A Make sure each H atom only as two dots surrounding it. To do this you will need the periodic table.
Sum the valence electrons from all the atoms.
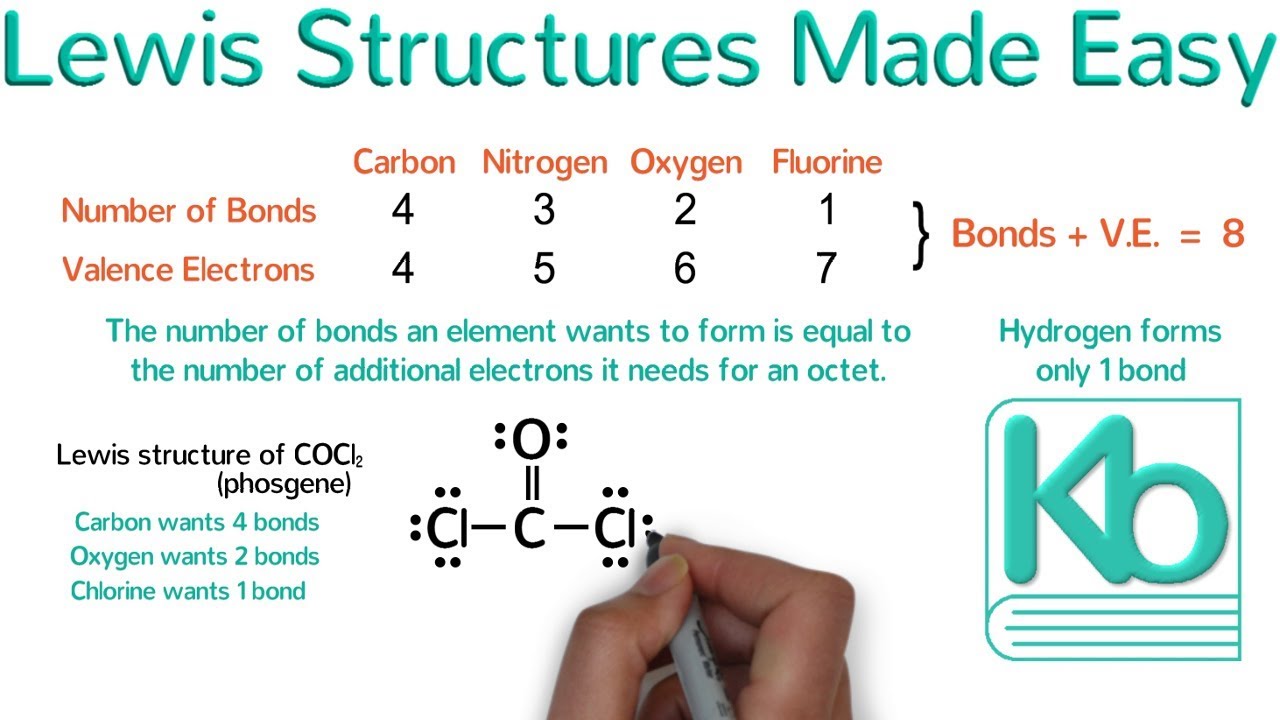
. General Rules for Drawing Covalently Bonded Lewis Structures 1. The general rules for writing Lewis structures include the following. Using the rules learned in class draw the Lewis structure of SeO2 and use it to answer the following questions.
If ionic treat each ion separately. Four electrons shared is a double bond and six electrons shared is a triple bond. Occurs when there are fewer than eight valence electrons around an atom resonance structures 3.
A lot of the time it is possible to draw more than on. The following rules are given to assist you. We have 4 dots left to use.
-Subtract one electron for each positive charge. Generally electrons are paired. Determine the total number of valence electrons in the molecule.
-Atoms that occur once or first are usually central. Shared pairs of electrons are drawn as lines between atoms while lone pairs of electrons are drawn as dots next to atoms. Use the Rest of the Dots to Finish Your Lewis Structure.
Determine the central atom and write the skeleton structure of the molecule. Lewis structures can be drawn with a set of general rules that give us a pretty good idea of how valence electrons are distributed around a molecule. Lewis structures for most molecules can be drawn by following a simple strategy.
General rules for writing LEWIS structures for molecules. It should be the most symmetric structure you can draw. For the most part Lewis structures reflect what is found when we gather experimental data for molecules in the laboratory.
-Add one electron for each negative charge. Worksheet on lewis structures 1 lewisstructureshwrk odt lewis structures homework draw the lewis structures for. In short these are the steps you need to follow for drawing a Lewis structure.
Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Compounds of low electronegativity metals with high electronegativity nonmetals ΔEN 16 are ionic as are compounds of metals with polyatomic anions. Rules for Drawing Lewis Structures For Molecular Compounds 1 Calculate the total number of electrons for the structure by summing the valence electrons of each atom in the molecule.
Write the correct skeletal structure for the molecule. There are two rules to follow when placing the remaining dots. Nitrogen 5 valence Phosphorus 10 valence.
How many valence electrons were used to make the drawing. One way to do this is to write the Lewis symbols for all of the atoms in the formula and count up all the dots. If the charge is negative add electrons and if the charge is positive subtract electrons Step 2.
Odd number of electrons. MUST FOLLOW IN ORDER. Count the total number of valence electrons in the structure Remember Group of Valence electrons 2.
How many lone pairs of electrons are around the central atom. Lewis electron dot Structures. Sometimes the number of valence e- is odd.
All atoms want to fulfill the octet rule. Unpaired electrons are observed in odd electron molecules such as NO and NO2. All valence electrons of the atoms in Lewis structures must be shown.
Rules for drawing Lewis structures. Determine whether the compound is covalent or ionic. A video tutorial for how to draw Lewis Structures in five steps.
Its not perfect but it is a useful tool for scientists trying to understand how molecules will react and their. Draw the initial skeletal structure with atoms connected by single bonds. Given a chemical formula corresponding to a molecule or molecular ion draw a Lewis structure.
When constructing a Lewis diagram keep in mind the octet rule which refers to the tendency of atoms to gain lose or share electrons until they are surrounded by eight valence electrons an octet. For a molecule uncharged that count is the correct. Hydrogen 2 valence Boron 6 valence Aluminum 6 valence Gallium 6 valence Indium 6 valence Sometimes Exceptions.
-Hydrogen cannot be central. The video covers the basic Lewis structures youll see in an introductory chemistry class. Make sure that H has only 1 bond and that the other atoms do not exceed their normal number 2 bonds to O 3 bonds to N 4 bonds to C and that no atom in the molecule has more than 4 bonds.
CBr 4-The least electronegative atom is central. Add one for each - charge extra electron. The central atom most of the time is the more positive atom.
B Make sure all other elements. Rules for Drawing Lewis Structures For molecules known to exist follow these rules to determine the Lewis structure. Determine the total number of valence electrons in the molecule or ion.
Add up the valence electrons of the atoms. Once the electron dot structure is complete translate this structure into a Lewis structure. Lewis Structures Worksheet Answers.
The Group number in the Periodic Table the number of valence electrons for an atom. Complete pairing of these e- is impossible and an octet around each atom cannot be achieved resonance structures 2. Two electrons are shared between two atoms constitutes a single bond.
Copyright 2001 L. Vsepr Chart Worksheet Answers Printable Worksheets and from bmwwiringtop Ionic bonding using. And recall that for main group elements the valence electrons can be determined from the group number.
Determine the total number of valence electrons in the molecule or polyatomic ionSimply. WRITING LEWIS DOT STRUCTURES Lewis structure or formula shows electrondot symbols for the atoms the bonding pairs as lines and the lone pairs that fill each atoms outer level valence shell as pairs of dots. The least electronegative atom will be central.
Determine the total number of valence electrons. Consult the molecular formula and sum up all the valence electrons from the separate atoms. LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1.
Write the skeletal structure. Subtract one for each charge missing electron. Lewis Structures Rules for Drawing Lewis Structure.
First of all a correct count of all valence electrons is essential. Less than an octet of valence electrons. Enter your answers as whole numbers not words 1.
Hydrogen atoms are always terminal only one bond Put more electronegative elements in terminal positions. In this video I explain the 3 rules that help us choose the correct Lewis structure for any molecule. If covalent treat the entire molecule.
Decide on a skeletal structure What is bonded to what The central atom is generally written first in the formula.

Electron Dot Model Of Bonding Lewis Structures
Lewis Electron Dot Diagrams Help
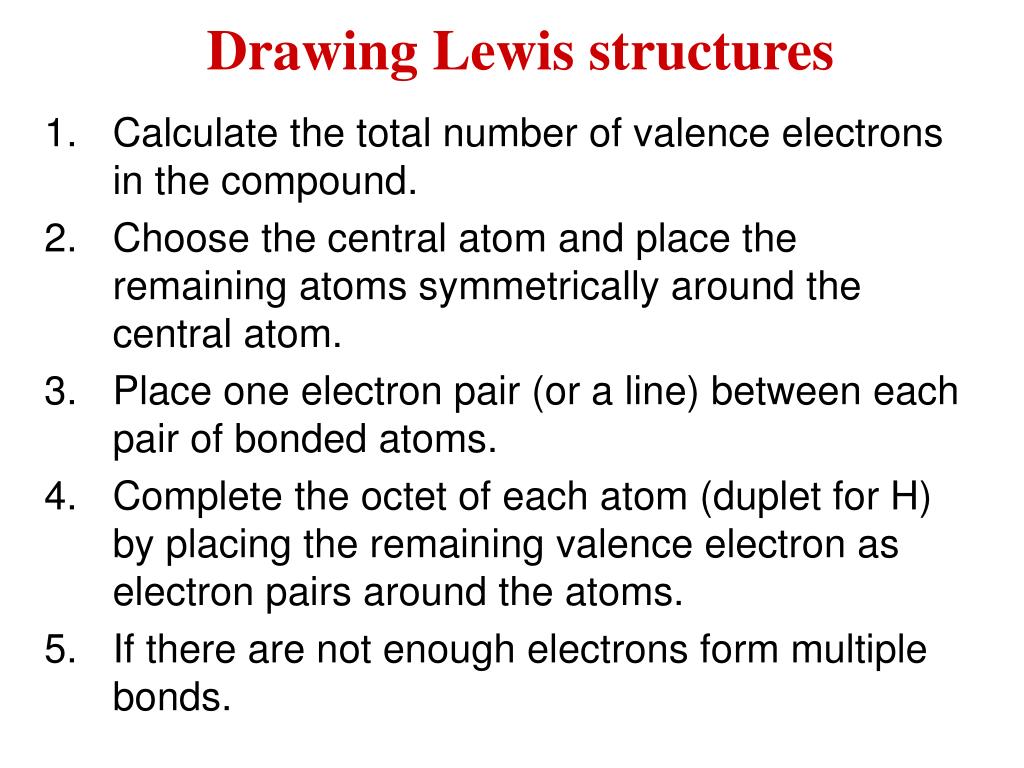
Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Youtube
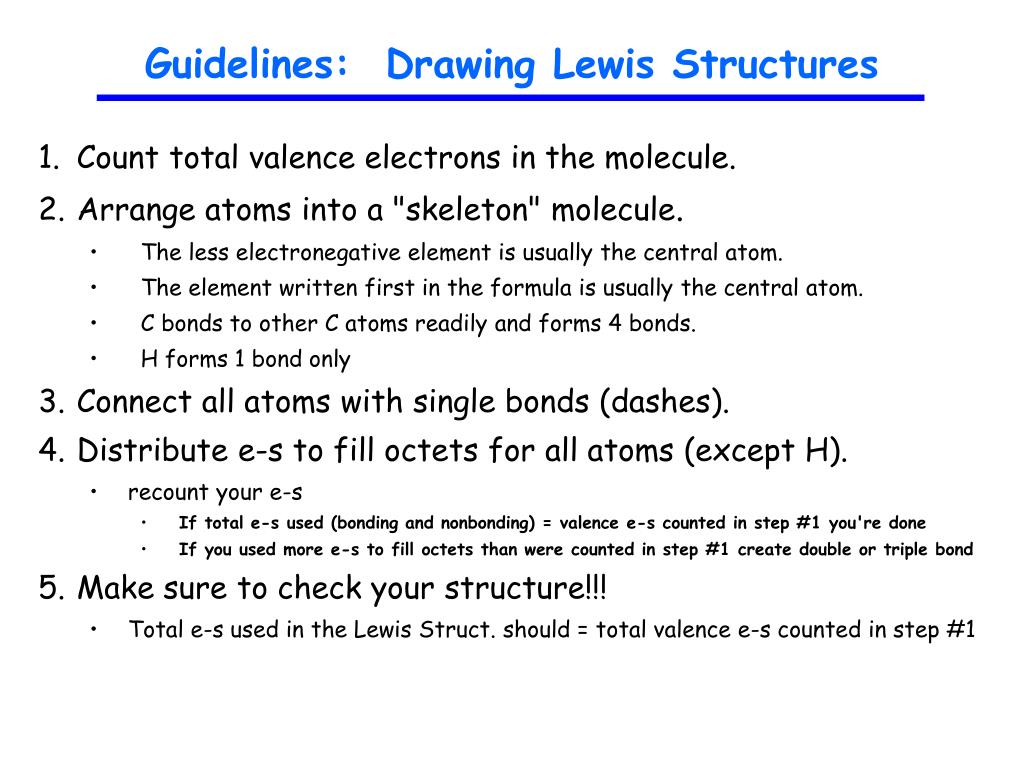
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949


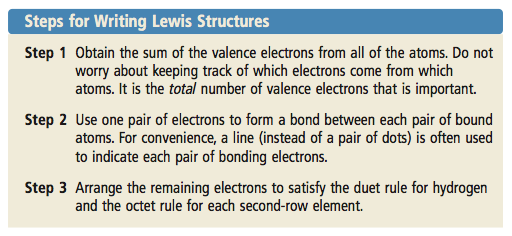

0 comments
Post a Comment